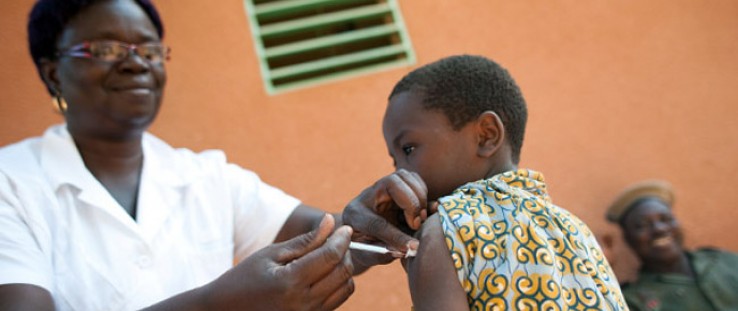
 Mass vaccination campaigns using the new vaccine reached nearly 20 million people in Burkina Faso, Mali, and Niger.
Gabe Bienczycki, PATH
Mass vaccination campaigns using the new vaccine reached nearly 20 million people in Burkina Faso, Mali, and Niger.
Gabe Bienczycki, PATH
 Mass vaccination campaigns using the new vaccine reached nearly 20 million people in Burkina Faso, Mali, and Niger.
Gabe Bienczycki, PATH
Mass vaccination campaigns using the new vaccine reached nearly 20 million people in Burkina Faso, Mali, and Niger.
Gabe Bienczycki, PATH
Florence, her youngest, was the first to fall ill, Rosalie Ouedraogo remembers. Only 6 years old, Florence escaped Group A meningococcal meningitis with her life, but the disease stole her hearing. During the next five years, meningitis came for Ouedraogo’s other children. First Xavier, who lost his hearing at age 15; and then Diane, at around 12.
In the West African country of Burkina Faso, where the Ouedraogos live, the threat of epidemic meningitis arrives with the dusty Harmattan winds every December and abates in May with the onset of what the mothers of sub-Saharan Africa call the “rains of hope.” The story of the effect of the disease on Rosalie Ouedraogo’s children is unusual only in that not one of her children was spared.
As the rainy season arrives this year, however, the stories from Burkina Faso are different. Widespread use of a new vaccine—developed through a unique collaboration in which USAID played a crucial part—has the potential to end the epidemics.
A Killer with Terrifying Speed
Bacterial meningitis is a serious infection of the thin lining surrounding the brain and spinal cord. Different types of bacteria can cause the disease. In the 25 countries of the African “meningitis belt,” which stretches from Senegal in the west to Ethiopia in the east, Group A Neisseria meningitis is the cause of almost all epidemics.
As many as 450 million people who live in the meningitis belt are at risk of brain damage, profound hearing loss, learning problems, or death from meningitis. Even with rapid diagnosis and treatment, 5-10 percent of people who become infected die, typically within a day or two of showing symptoms. In the most remote communities of sub-Saharan Africa, where people do not receive timely medical attention, the fatality rate can approach 100 percent.
When the largest epidemic ever recorded ravaged the region in 1996 and 1997, more than 250,000 people became ill. At least 25,000 died.
Quest for a Better Vaccine
The 1996-1997 epidemic was a wake-up call for the international health community. After a century of recurrent meningitis outbreaks and 30 years relying on selective use of a vaccine not wholly suited to conditions in Africa, the epidemics were growing more deadly. African public health officials approached the World Health Organization (WHO) and asked for help in finding a solution.
By 2000, health leaders, vaccine manufacturers, and scientists brought together by WHO concluded that the development of a new conjugate vaccine to fight epidemic meningitis in Africa was a priority. Previous research showed that conjugate vaccines, where polysaccharides are linked to carrier proteins, had potential to provide longer-lasting protection than polysaccharide antigens.
A year later, the Bill & Melinda Gates Foundation provided a 10-year grant to establish the Meningitis Vaccine Project (MVP), a partnership between PATH, a Seattle-based NGO that works on health issues in developing countries, and WHO to lead the development, testing, licensure, and widespread introduction of a conjugate vaccine with the potential to protect millions from epidemic meningitis.
Finding an Affordable Solution
The challenge facing the new project was daunting. How would a partnership between two public health organizations develop this new vaccine? Who would manufacture the vaccine, and where would it be tested? How would MVP navigate international regulatory processes? Moreover, how would it be able to develop and deliver a vaccine at a price that the countries of the meningitis belt could afford—less than 50 cents a dose?
“The answers,” says PATH President and CEO Christopher J. Elias, “came through innovative partnerships that spanned four continents and provide a model for affordable vaccine development.”
The Serum Institute of India, the world’s largest manufacturer of measles and diphtheria-tetanus-pertussis vaccines, agreed to make the new meningitis vaccine for less than 50 cents. Synco BioPartners in the Netherlands and the Serum Institute provided raw materials for the vaccine. The U.S. National Institutes of Health helped transfer the conjugation technology, developed by scientists at the U.S. Food and Drug Administration, to the Serum Institute at almost no cost. And, a network of experts, including senior African public health officials, helped design clinical trials and establish African trial sites.
Thinking Outside the Global Budget
As the vaccine manufacturing and regulatory processes progressed, other partners came forward with support, including USAID. Since 2006, the Agency has provided $1.2 million for a variety of activities that made development and delivery of the vaccine possible.
USAID funding allowed MVP to pursue activities that could not be funded in any other way.
With USAID funding, the project was able to do a comprehensive study of the economic costs of meningitis epidemics that helped make the case for the vaccine. Agency funds provided for better disease surveillance in Burkina Faso, Mali, and Niger, three of the countries most severely affected by meningitis and the first to receive the vaccine.
The Agency’s contribution provided training for African microbiologists assigned to regional laboratories, and allowed African scientists and epidemiologists to travel to Pune, India—home of the vaccine’s manufacturer—to review the development, clinical evaluation, and planned introduction of the new vaccine.
Susan McKinney, senior technical adviser for immunization at USAID, noted, “The Agency helped support training and consultative activities for the Drugs Controller General of India, the national authority that regulates market authorization of vaccines developed in India, thus paving the way for vaccine introduction in Africa.”
A Beginning to the End
By late 2010, the new vaccine, called MenAfriVac™, was ready for introduction. MenAfriVac™ was developed at less than one-tenth of the $500 million typically needed to bring a new vaccine to market—a major cost savings. In addition, the reduction in meningitis cases is expected to free up significant funds that countries can use to address other public health problems.
In December 2010, children and young adults in Burkina Faso, Mali, and Niger began to receive the vaccine. By the end of the month, nearly 20 million people, ages 1 through 29, had been vaccinated against epidemic meningitis with the new vaccine.
The early impact analysis following introduction is very encouraging; there have been no Group A meningococcal outbreaks in Burkina Faso, Mali, and Niger.
The challenge ahead is to make sure the vaccine reaches the rest of the meningitis belt. WHO estimates that MenAfriVac™ can save more than 140,000 lives and prevent more than 240,000 permanent disabilities over the course of a decade if it gets to the 300 million more children and young adults who need it. But even at a cost of less than 50 cents a dose, it will take an additional $475 million to protect them. Support is still needed.
WHO and PATH are working to build support for sustaining the immunization effort and expanding it to the 22 countries where people have yet to receive the vaccine. USAID is committed to vaccine introduction and is a major supporter of GAVI, the international agency responsible for much of the introduction funding.
If the immunization campaign is successful, the history of the African meningitis belt will change again, this time for the better. On that day, families like the Ouedraogos will no longer live in fear when the wind begins to blow.
Dr. F. Marc LaForce is director of the Meningitis Vaccine Project.
For his work with the Meningitis Vaccine Project, LaForce was recently nominated as one of Time magazine's most influential people in the world for 2011.












Comment
Make a general inquiry or suggest an improvement.